| NASDAQ: PMN Targeting the underlying cause of neurodegenerative diseases |
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Legal Disclaimers This slide deck may contain certain forward-looking information. Such information involves known and unknown risks, uncertainties and other factors that may cause actual results, performance or achievements to be materially different from those implied by statements herein, and therefore these statements should not be read as guarantees of future performance or results. All forward-looking statements are based on ProMIS Neurosciences Inc.’s (the “Company”) current beliefs as well as assumptions made by and information currently available to it, as well as other factors. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this slide deck. Due to risks and uncertainties, including the risks and uncertainties identified by the Company in its public securities filings available online at sec.gov, actual events may differ materially from current expectations. The Company disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise. 2 |
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Unique potential in areas of great unmet need Clinical-stage biopharma with pipeline selectively targeting specific, disease-causing misfolded proteins 3 • Unique selectivity may create potential to address the unmet need for safer, more efficacious therapies • Significant market potential across a range of neurodegenerative diseases • Seasoned leadership team with global development and deep domain experience • Funding to strive to hit milestones, with up to $122.7 million secured from PIPE in July 2024 from leading healthcare specialty funds |
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PRECISE-AD: Lead program PMN310 in ongoing placebo-controlled Phase 1b clinical study in Alzheimer’s patients 4 Humanized monoclonal antibody designed to bind toxic amyloid-beta oligomers (AbOs), NOT monomers or plaques Phase 1a: PMN310 was well-tolerated in healthy volunteers, crossed the blood-brain barrier and achieved concentrations suggesting sufficient target engagement with a half-life supportive of monthly dosing PRECISE-AD: Phase 1b clinical trial is ongoing in AD patients. 12-month endpoints include clinical outcomes, safety (incidence of ARIA) and biomarkers Broad pre-clinical pipeline: Includes antibody and vaccine candidates targeting ALS, MSA, Parkinson’s, Dementia with Lewy bodies, and others |
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Experienced Leadership Team 5 Josh Mandel-Brehm, M.B.A. Independent Director Maggie Shafmaster, Ph.D., J.D. Lead Independent Director Neil K. Warma, M.B.A., B.Sc. Chief Executive Officer William Wyman, M.B.A. Independent Director Patrick Kirwin, B.A., J.D. Independent Director Executive Management Eugene Williams, M.B.A. Chairman and Co-founder Neil Cashman, M.D. Chief Scientific Officer and Co-founder Gavin Malenfant Chief Operating Officer David Wishart, Ph.D. Chief Physics Officer Neil Cashman, M.D. Chief Scientific Officer Dan Geffken Chief Financial Officer Johanne Kaplan, Ph.D. Chief Development Officer Larry Altstiel, M.D., Ph.D. Chief Medical Officer Neil K. Warma Chief Executive Officer Board of Directors Clinical Advisory Board Henrik Zetterberg, MD, PhD University of Gothenburg, Sweden Dr. Michael Weiner UCSF, San Francisco, CA Howard Fillit, MD ADDF, New York, NY Suzanne Hendrix, PhD Pentara, Millcreek, UT |
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Lead programs targeting growing opportunities in neurodegeneration 6 Clinical Phase 1b Alzheimer’s Disease (AD) Amyotrophic Lateral Sclerosis (ALS) Synucleinopathies (MSA, PD, DLB, etc.)* *MSA - Multiple system atrophy; PD - Parkinson’s disease; DLB: Dementia with Lewy bodies 1Alzheimer’s Association 2024 AD Facts and Figures, Alzheimer's Disease Facts and Figures 2Arthur et al, 2016, Nature Communications 12.7 M1 Expected by 2050 6.9 M People with AD in U.S today Unmet Needs Opportunity Selectivity Targets toxic amyloid-beta (Aβ) oligomers, believed to be the key driver of AD progression Selectively targets pathogenic cytoplasmic TDP-43 aggregates to preserve the function of normal TDP-43 Despite new therapies, lack of broad efficacy and safety concerns remain Targets toxic alpha-synuclein oligomers and small soluble fibrils, does not bind physiologic monomers and tetramers Uniformly fatal illness with limited effective treatment Increasing prevalence with over 376,000 cases worldwide by 20402 Symptomatic treatment but no disease modifying therapies for PD. No effective treatment for MSA and DLB Growth driven by increasing prevalence and awareness, aging population |
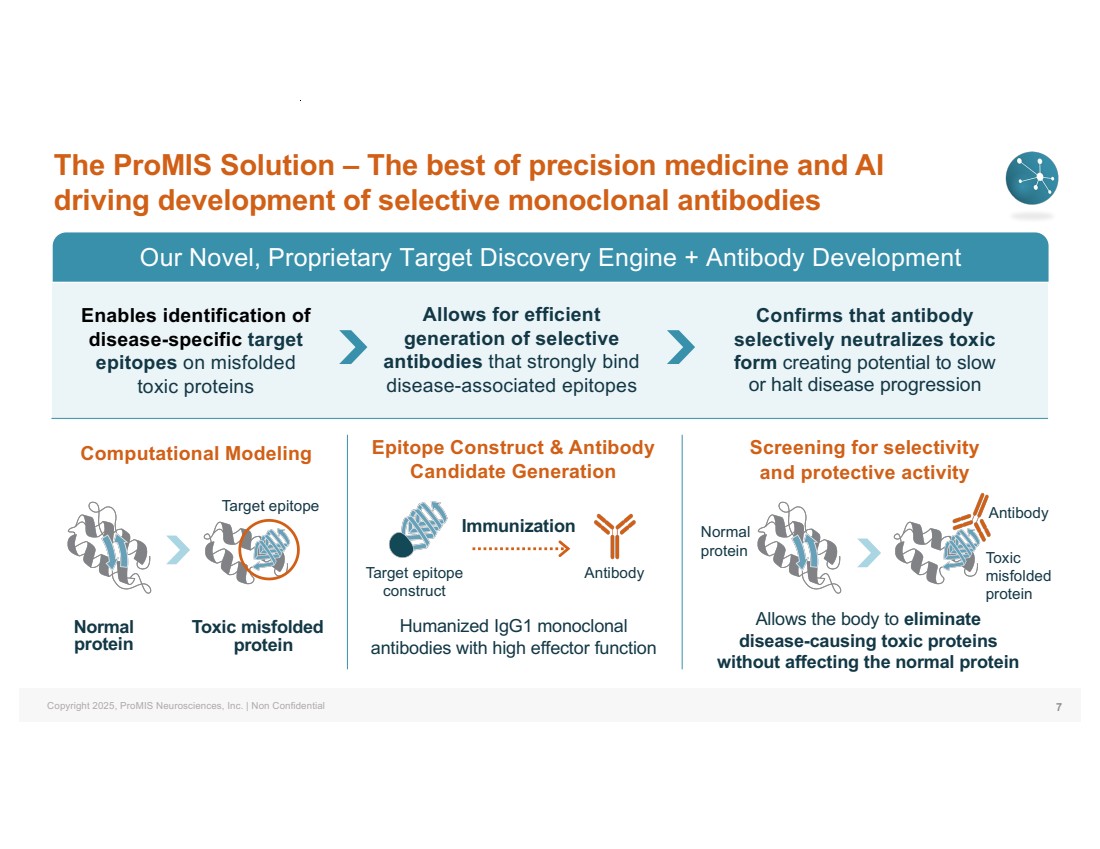
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential The ProMIS Solution – The best of precision medicine and AI driving development of selective monoclonal antibodies Our Novel, Proprietary Target Discovery Engine + Antibody Development Computational Modeling Epitope Construct & Antibody Candidate Generation Normal protein Toxic misfolded protein Screening for selectivity and protective activity Confirms that antibody selectively neutralizes toxic form creating potential to slow or halt disease progression Enables identification of disease-specific target epitopes on misfolded toxic proteins Allows for efficient generation of selective antibodies that strongly bind disease-associated epitopes 7 Allows the body to eliminate disease-causing toxic proteins without affecting the normal protein Humanized IgG1 monoclonal antibodies with high effector function Target epitope Immunization Target epitope construct Antibody Normal protein Toxic misfolded protein Antibody |
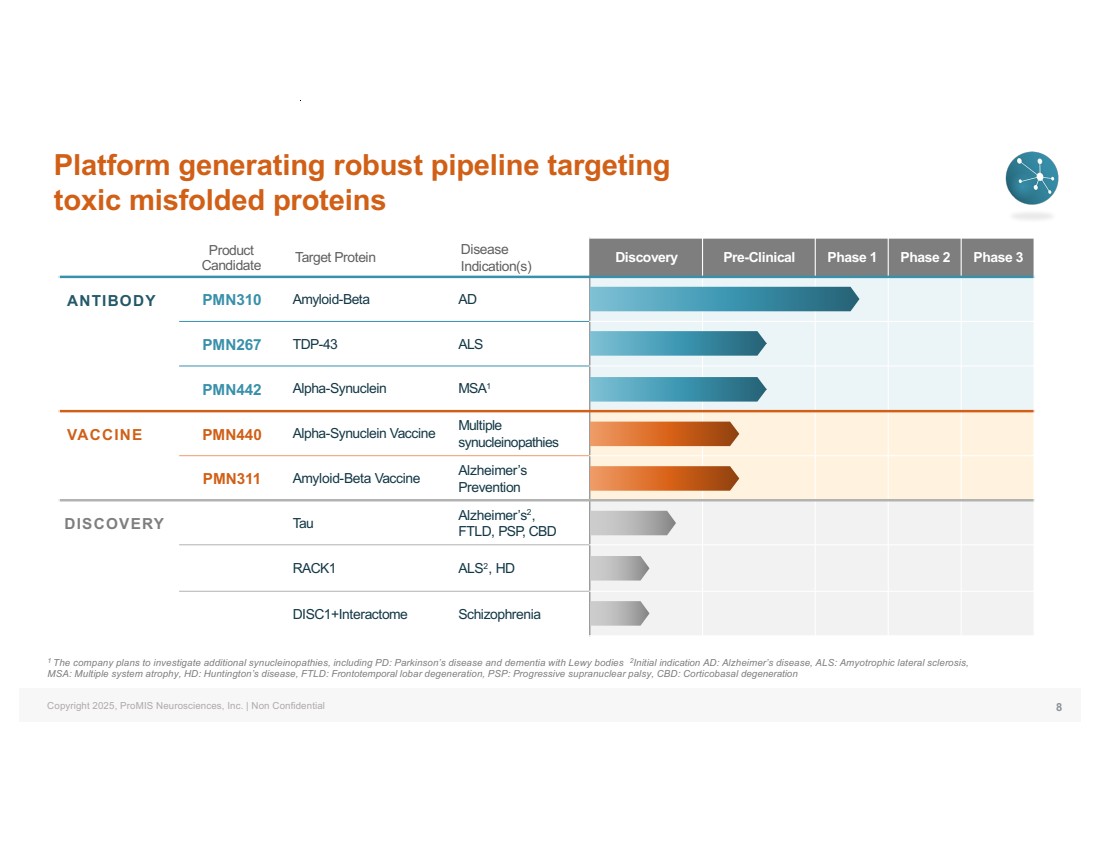
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Product Candidate Target Protein Disease Indication(s) Discovery Pre-Clinical Phase 1 Phase 2 Phase 3 ANTIBODY PMN310 Amyloid-Beta AD PMN267 TDP-43 ALS PMN442 Alpha-Synuclein MSA1 VACCINE PMN440 Alpha-Synuclein Vaccine Multiple synucleinopathies PMN311 Amyloid-Beta Vaccine Alzheimer’s Prevention DISCOVERY Tau Alzheimer’s2, FTLD, PSP, CBD RACK1 ALS2, HD DISC1+Interactome Schizophrenia 1 The company plans to investigate additional synucleinopathies, including PD: Parkinson’s disease and dementia with Lewy bodies 2Initial indication AD: Alzheimer’s disease, ALS: Amyotrophic lateral sclerosis, MSA: Multiple system atrophy, HD: Huntington’s disease, FTLD: Frontotemporal lobar degeneration, PSP: Progressive supranuclear palsy, CBD: Corticobasal degeneration Platform generating robust pipeline targeting toxic misfolded proteins 8 |
| Lead Clinical Candidate PMN310 in Alzheimer’s Disease Selectivity for Toxic Aβ Oligomers |
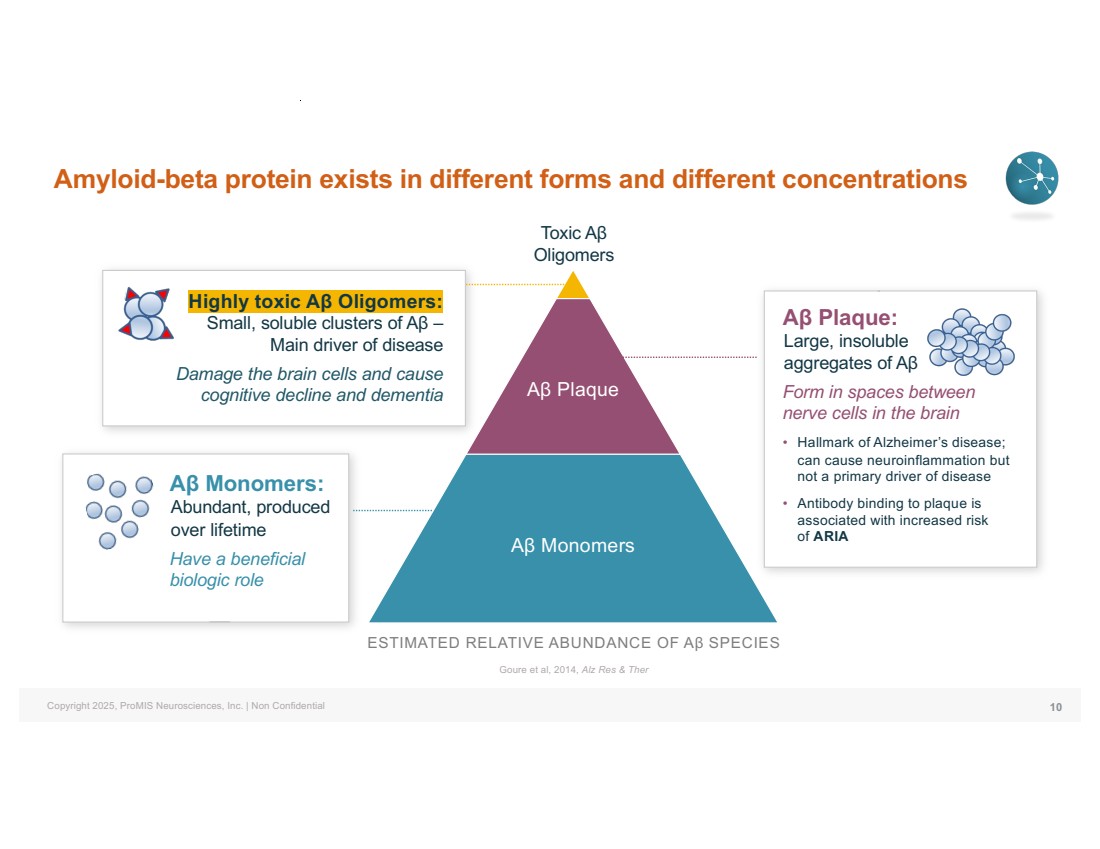
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Amyloid-beta protein exists in different forms and different concentrations 10 Aβ Monomers: Abundant, produced over lifetime Have a beneficial biologic role Highly toxic Aβ Oligomers: Small, soluble clusters of Aβ – Main driver of disease Damage the brain cells and cause cognitive decline and dementia ESTIMATED RELATIVE ABUNDANCE OF Aβ SPECIES Goure et al, 2014, Alz Res & Ther Toxic Aβ Oligomers Aβ Plaque: Large, insoluble aggregates of Aβ Form in spaces between nerve cells in the brain • Hallmark of Alzheimer’s disease; can cause neuroinflammation but not a primary driver of disease • Antibody binding to plaque is associated with increased risk of ARIA Aβ Plaque Aβ Monomers |
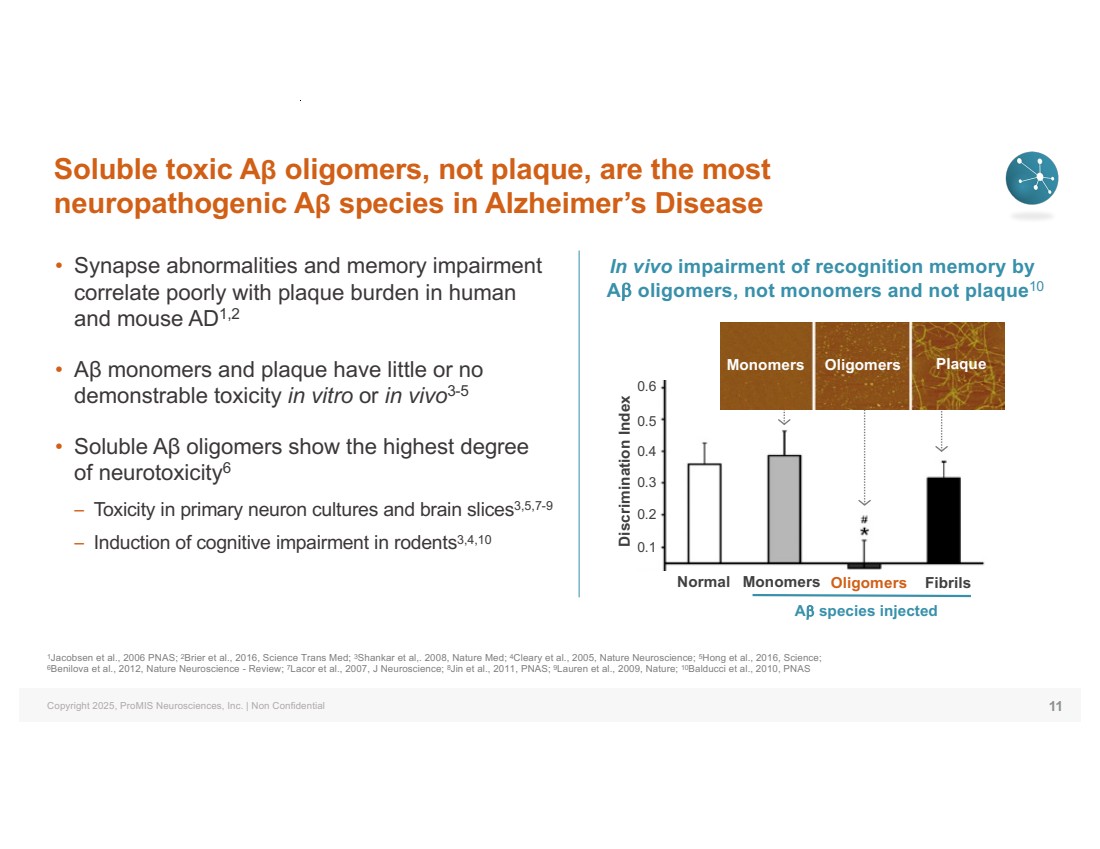
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Soluble toxic Aβ oligomers, not plaque, are the most neuropathogenic Aβ species in Alzheimer’s Disease • Synapse abnormalities and memory impairment correlate poorly with plaque burden in human and mouse AD1,2 • Aβ monomers and plaque have little or no demonstrable toxicity in vitro or in vivo3-5 • Soluble Aβ oligomers show the highest degree of neurotoxicity6 – Toxicity in primary neuron cultures and brain slices3,5,7-9 – Induction of cognitive impairment in rodents3,4,10 11 1Jacobsen et al., 2006 PNAS; 2Brier et al., 2016, Science Trans Med; 3Shankar et al,. 2008, Nature Med; 4Cleary et al., 2005, Nature Neuroscience; 5Hong et al., 2016, Science; 6Benilova et al., 2012, Nature Neuroscience - Review; 7Lacor et al., 2007, J Neuroscience; 8Jin et al., 2011, PNAS; 9Lauren et al., 2009, Nature; 10Balducci et al., 2010, PNAS In vivo impairment of recognition memory by Ab oligomers, not monomers and not plaque10 Normal Monomers Oligomers Fibrils Ab species injected Monomers Oligomers Plaque Discrimination Index 0.6 0.5 0.4 0.3 0.2 0.1 |
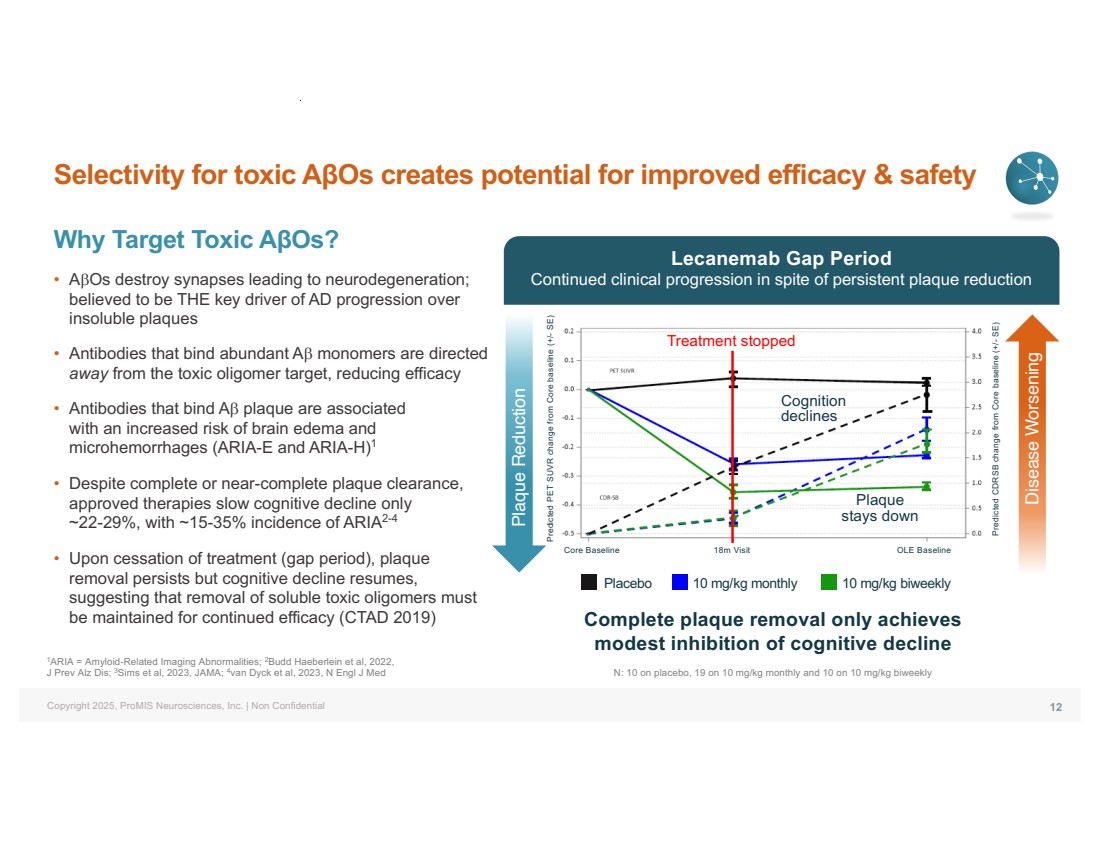
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Selectivity for toxic AβOs creates potential for improved efficacy & safety Why Target Toxic AβOs? • AbOs destroy synapses leading to neurodegeneration; believed to be THE key driver of AD progression over insoluble plaques • Antibodies that bind abundant Ab monomers are directed away from the toxic oligomer target, reducing efficacy • Antibodies that bind Ab plaque are associated with an increased risk of brain edema and microhemorrhages (ARIA-E and ARIA-H)1 • Despite complete or near-complete plaque clearance, approved therapies slow cognitive decline only ~22-29%, with ~15-35% incidence of ARIA2-4 • Upon cessation of treatment (gap period), plaque removal persists but cognitive decline resumes, suggesting that removal of soluble toxic oligomers must be maintained for continued efficacy (CTAD 2019) 12 1ARIA = Amyloid-Related Imaging Abnormalities; 2Budd Haeberlein et al, 2022, J Prev Alz Dis; 3Sims et al, 2023, JAMA; 4van Dyck et al, 2023, N Engl J Med Complete plaque removal only achieves modest inhibition of cognitive decline Treatment stopped Cognition declines Plaque stays down N: 10 on placebo, 19 on 10 mg/kg monthly and 10 on 10 mg/kg biweekly Placebo 10 mg/kg monthly 10 mg/kg biweekly Predicted PET SUVR change from Core baseline (+/- SE) Plaque Reduction Core Baseline Predicted CDRSB change from Core baseline (+/- SE) Lecanemab Gap Period Continued clinical progression in spite of persistent plaque reduction Disease Worsening 18m Visit OLE Baseline |
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Specific targeting of toxic Aβ oligomers is needed for increased efficacy and improved safety profile Avoiding off-target delivery of drug, to more plentiful monomers and plaque • Most drugs cross-react with several species of Ab • Less drug getting to oligomer target potentially reducing efficacy and increasing side effects Selective targeting of only the toxic oligomer species • Needed to improve efficacy AND avoid ARIA • May allow for lower dosing (drug is not misdirected) PMN310 designed to selectively bind Aβ oligomers without binding monomers or plaque • Selectivity for toxic oligomers without monomer distraction may increase clinical activity • Avoidance of plaque may carry a reduced risk of ARIA The Challenge The Potential Solution 1 2 13 |
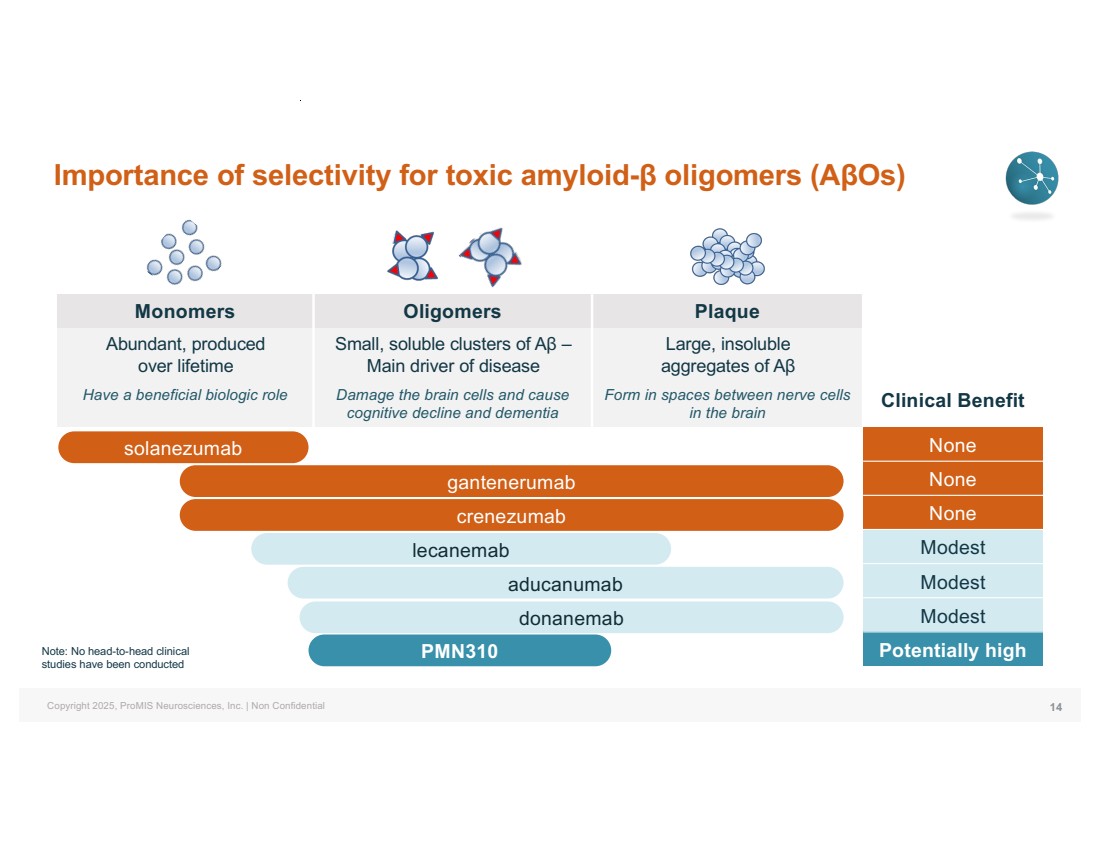
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Importance of selectivity for toxic amyloid-β oligomers (AβOs) 14 Monomers Oligomers Plaque Abundant, produced over lifetime Have a beneficial biologic role Small, soluble clusters of Aβ – Main driver of disease Damage the brain cells and cause cognitive decline and dementia Large, insoluble aggregates of Aβ Form in spaces between nerve cells in the brain Clinical Benefit None None None Modest Modest Modest Potentially high solanezumab gantenerumab crenezumab lecanemab aducanumab donanemab Note: No head-to-head clinical PMN310 studies have been conducted |
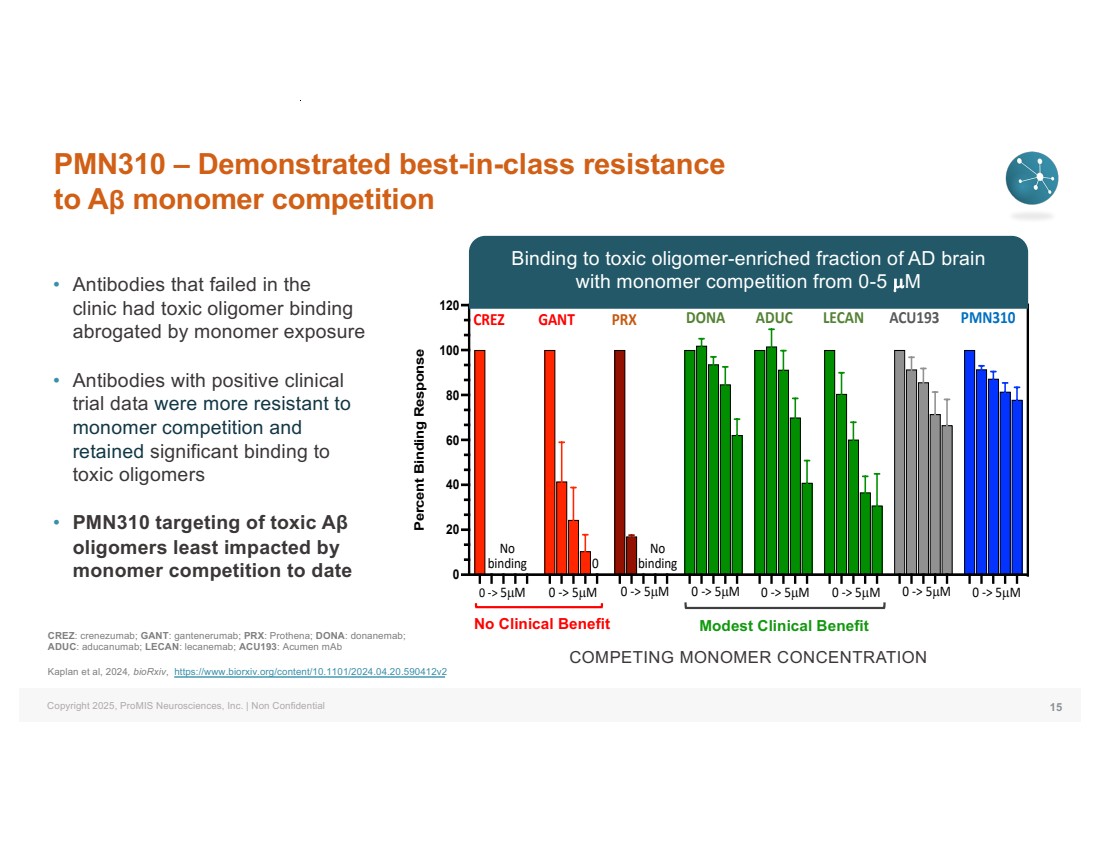
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PMN310 – Demonstrated best-in-class resistance to Aβ monomer competition • Antibodies that failed in the clinic had toxic oligomer binding abrogated by monomer exposure • Antibodies with positive clinical trial data were more resistant to monomer competition and retained significant binding to toxic oligomers • PMN310 targeting of toxic Aβ oligomers least impacted by monomer competition to date 15 CREZ GANT PRX DONA ADUC LECAN ACU193 PMN310 0 -> 5µM 0 -> 5µM 0 -> 5µM 0 -> 5µM 0 -> 5µM 0 -> 5µM No binding 0 -> 5µM 0 No binding 0 -> 5µM 0 20 40 60 80 100 120 Percent Binding Response No Clinical Benefit Modest Clinical Benefit COMPETING MONOMER CONCENTRATION CREZ: crenezumab; GANT: gantenerumab; PRX: Prothena; DONA: donanemab; ADUC: aducanumab; LECAN: lecanemab; ACU193: Acumen mAb Kaplan et al, 2024, bioRxiv, https://www.biorxiv.org/content/10.1101/2024.04.20.590412v2 Binding to toxic oligomer-enriched fraction of AD brain with monomer competition from 0-5 µM |
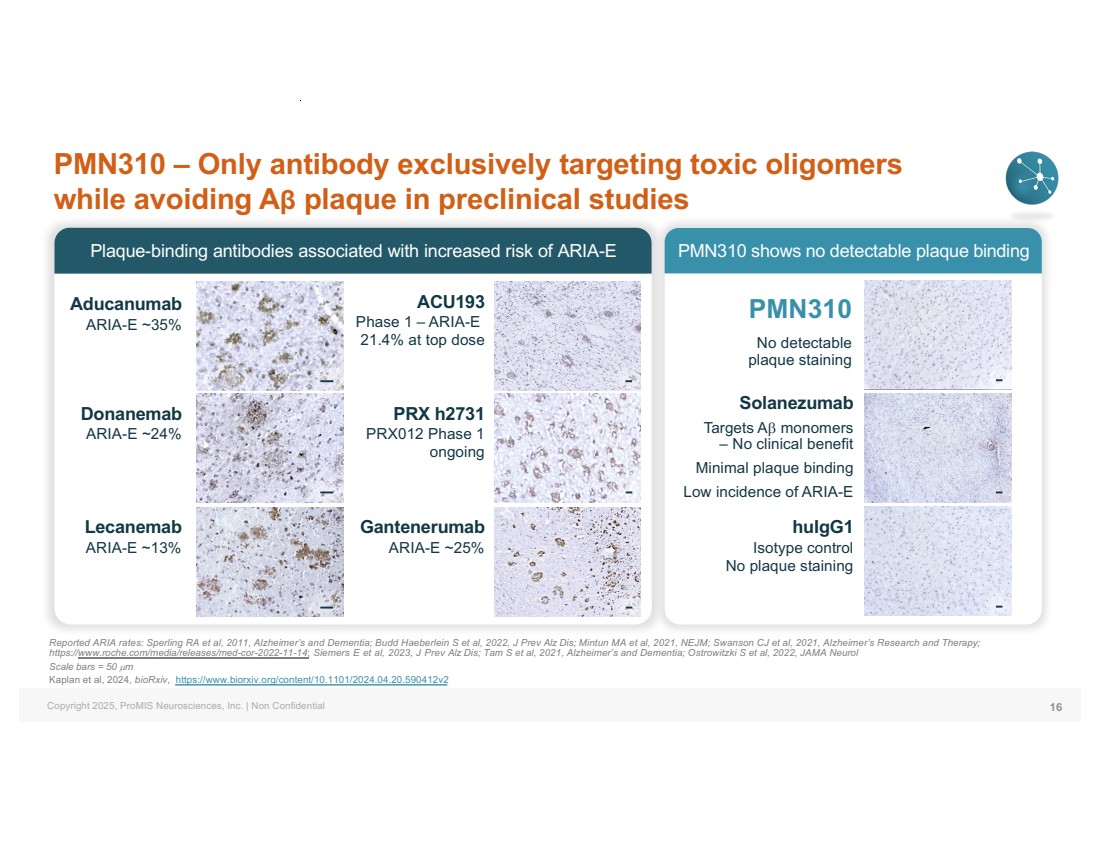
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Plaque-binding antibodies associated with increased risk of ARIA-E PMN310 shows no detectable plaque binding PMN310 – Only antibody exclusively targeting toxic oligomers while avoiding Aβ plaque in preclinical studies 16 Reported ARIA rates: Sperling RA et al, 2011, Alzheimer’s and Dementia; Budd Haeberlein S et al, 2022, J Prev Alz Dis; Mintun MA et al, 2021, NEJM; Swanson CJ et al, 2021, Alzheimer’s Research and Therapy; https://www.roche.com/media/releases/med-cor-2022-11-14; Siemers E et al, 2023, J Prev Alz Dis; Tam S et al, 2021, Alzheimer’s and Dementia; Ostrowitzki S et al, 2022, JAMA Neurol Scale bars = 50 µm Kaplan et al, 2024, bioRxiv, https://www.biorxiv.org/content/10.1101/2024.04.20.590412v2 PMN310 No detectable plaque staining Gantenerumab ARIA-E ~25% huIgG1 Isotype control No plaque staining Aducanumab ARIA-E ~35% Donanemab ARIA-E ~24% Lecanemab ARIA-E ~13% Solanezumab Targets Ab monomers – No clinical benefit Minimal plaque binding Low incidence of ARIA-E ACU193 Phase 1 – ARIA-E 21.4% at top dose PRX h2731 PRX012 Phase 1 ongoing |
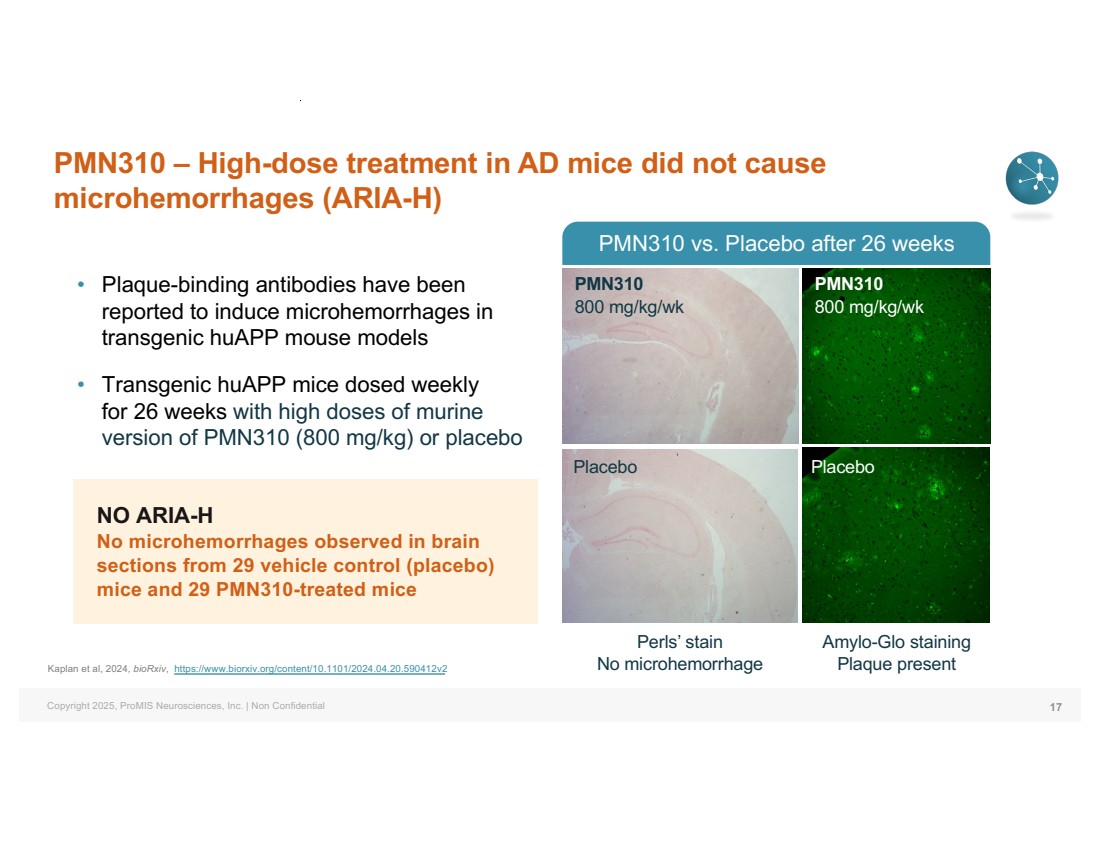
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential NO ARIA-H No microhemorrhages observed in brain sections from 29 vehicle control (placebo) mice and 29 PMN310-treated mice PMN310 – High-dose treatment in AD mice did not cause microhemorrhages (ARIA-H) 17 • Plaque-binding antibodies have been reported to induce microhemorrhages in transgenic huAPP mouse models • Transgenic huAPP mice dosed weekly for 26 weeks with high doses of murine version of PMN310 (800 mg/kg) or placebo Perls’ stain No microhemorrhage Amylo-Glo staining Plaque present PMN310 vs. Placebo after 26 weeks Placebo Placebo PMN310 800 mg/kg/wk PMN310 800 mg/kg/wk Kaplan et al, 2024, bioRxiv, https://www.biorxiv.org/content/10.1101/2024.04.20.590412v2 |
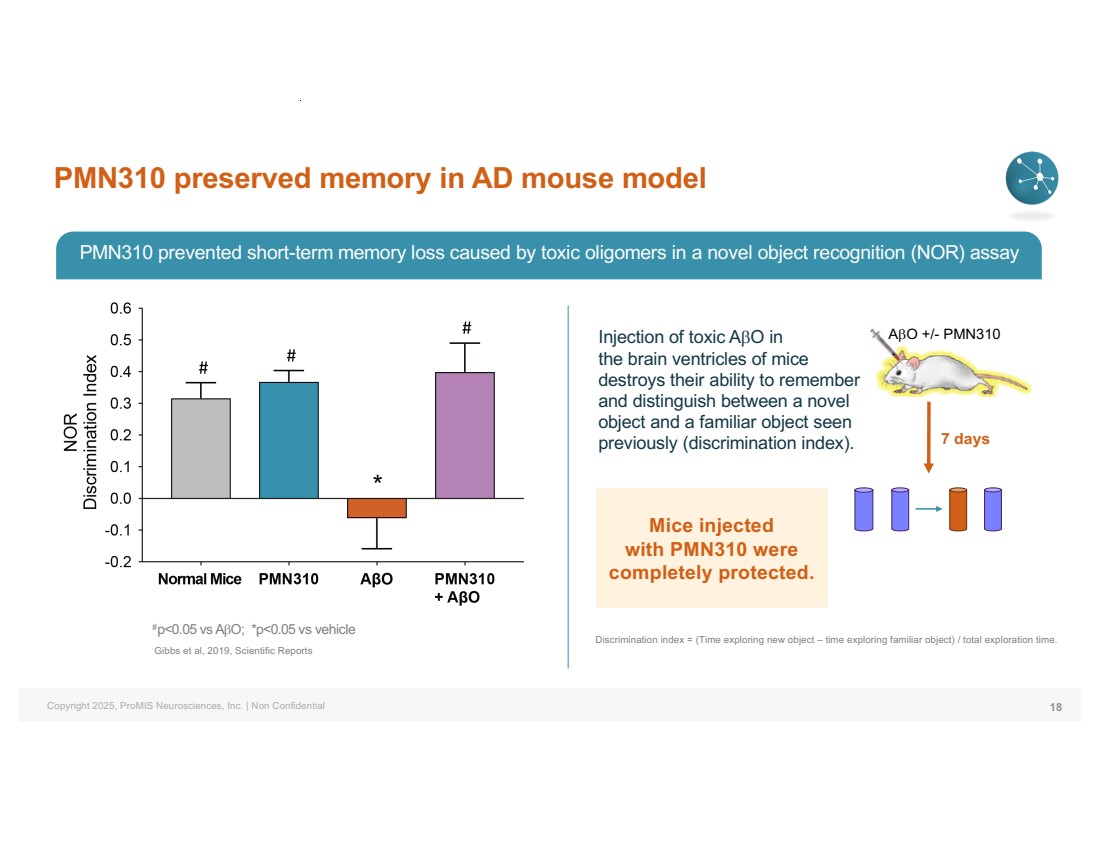
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PMN310 preserved memory in AD mouse model 18 #p<0.05 vs AbO; *p<0.05 vs vehicle Gibbs et al, 2019, Scientific Reports AbO +/- PMN310 7 days Injection of toxic AbO in the brain ventricles of mice destroys their ability to remember and distinguish between a novel object and a familiar object seen previously (discrimination index). Discrimination index = (Time exploring new object – time exploring familiar object) / total exploration time. PMN310 prevented short-term memory loss caused by toxic oligomers in a novel object recognition (NOR) assay Mice injected with PMN310 were completely protected. |
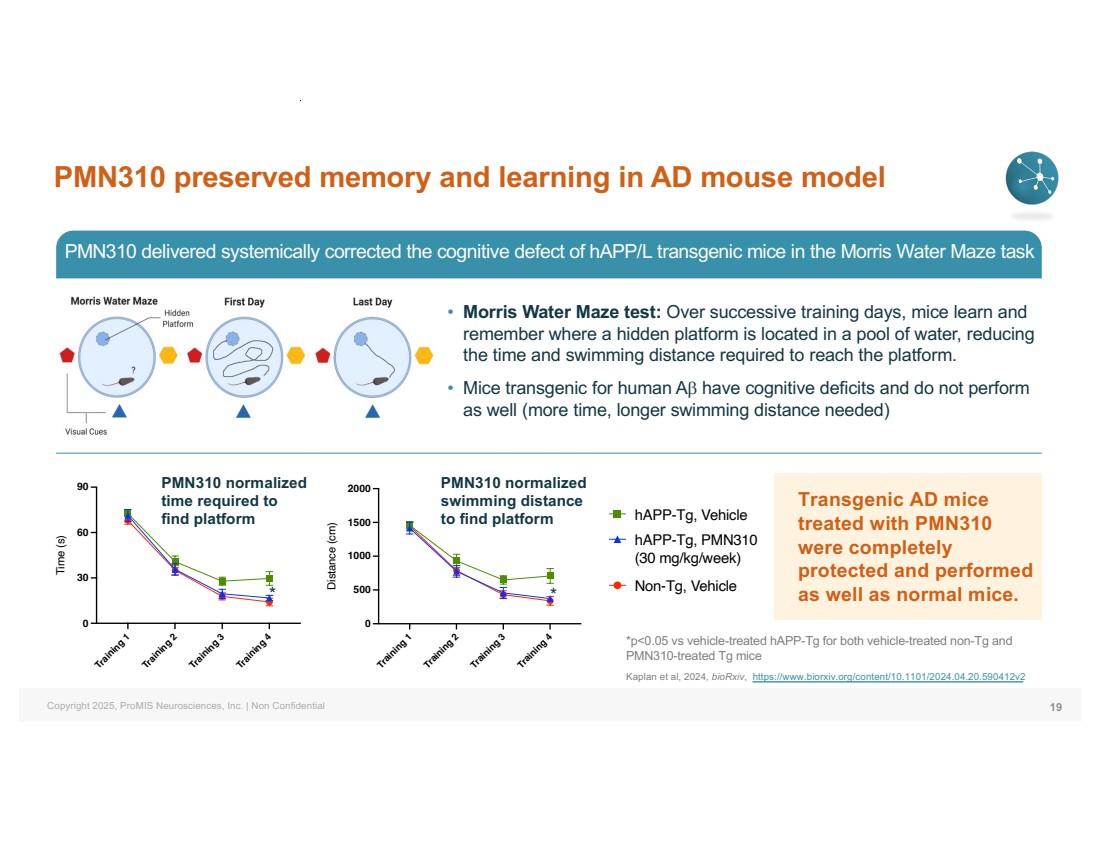
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PMN310 preserved memory and learning in AD mouse model 19 *p<0.05 vs vehicle-treated hAPP-Tg for both vehicle-treated non-Tg and PMN310-treated Tg mice Kaplan et al, 2024, bioRxiv, https://www.biorxiv.org/content/10.1101/2024.04.20.590412v2 Training 1 Training 2 Training 3 Training 4 0 500 1000 1500 2000 Distance (cm) Non-Tg, Vehicle hAPP-Tg, Vehicle hAPP-Tg, PMN310 (30 mg/kg/week) Training 1 Training 2 Training 3 Training 4 0 30 60 90 Time (s) * PMN310 normalized time required to find platform Training 1 Training 2 Training 3 Training 4 0 500 1000 1500 2000 Distance (cm) * PMN310 normalized swimming distance to find platform • Morris Water Maze test: Over successive training days, mice learn and remember where a hidden platform is located in a pool of water, reducing the time and swimming distance required to reach the platform. • Mice transgenic for human Ab have cognitive deficits and do not perform as well (more time, longer swimming distance needed) PMN310 delivered systemically corrected the cognitive defect of hAPP/L transgenic mice in the Morris Water Maze task Transgenic AD mice treated with PMN310 were completely protected and performed as well as normal mice. |
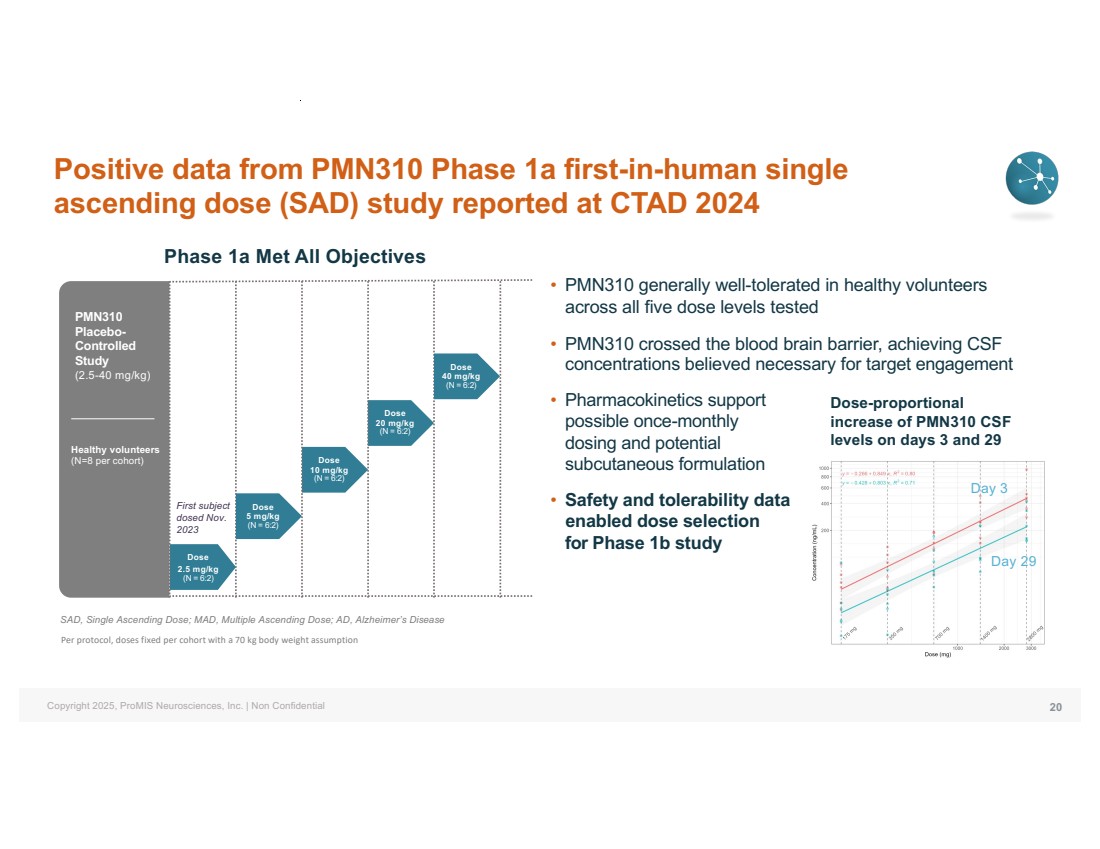
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Healthy volunteers (N=8 per cohort) First subject dosed Nov. 2023 Dose 2.5 mg/kg (N = 6:2) Dose 5 mg/kg (N = 6:2) Dose 10 mg/kg (N = 6:2) Dose 20 mg/kg (N = 6:2) Dose 40 mg/kg (N = 6:2) PMN310 Placebo-Controlled Study (2.5-40 mg/kg) Positive data from PMN310 Phase 1a first-in-human single ascending dose (SAD) study reported at CTAD 2024 • PMN310 generally well-tolerated in healthy volunteers across all five dose levels tested • PMN310 crossed the blood brain barrier, achieving CSF concentrations believed necessary for target engagement • Pharmacokinetics support possible once-monthly dosing and potential subcutaneous formulation • Safety and tolerability data enabled dose selection for Phase 1b study SAD, Single Ascending Dose; MAD, Multiple Ascending Dose; AD, Alzheimer’s Disease Per protocol, doses fixed per cohort with a 70 kg body weight assumption Dose-proportional increase of PMN310 CSF levels on days 3 and 29 Day 3 Day 29 Phase 1a Met All Objectives 20 |
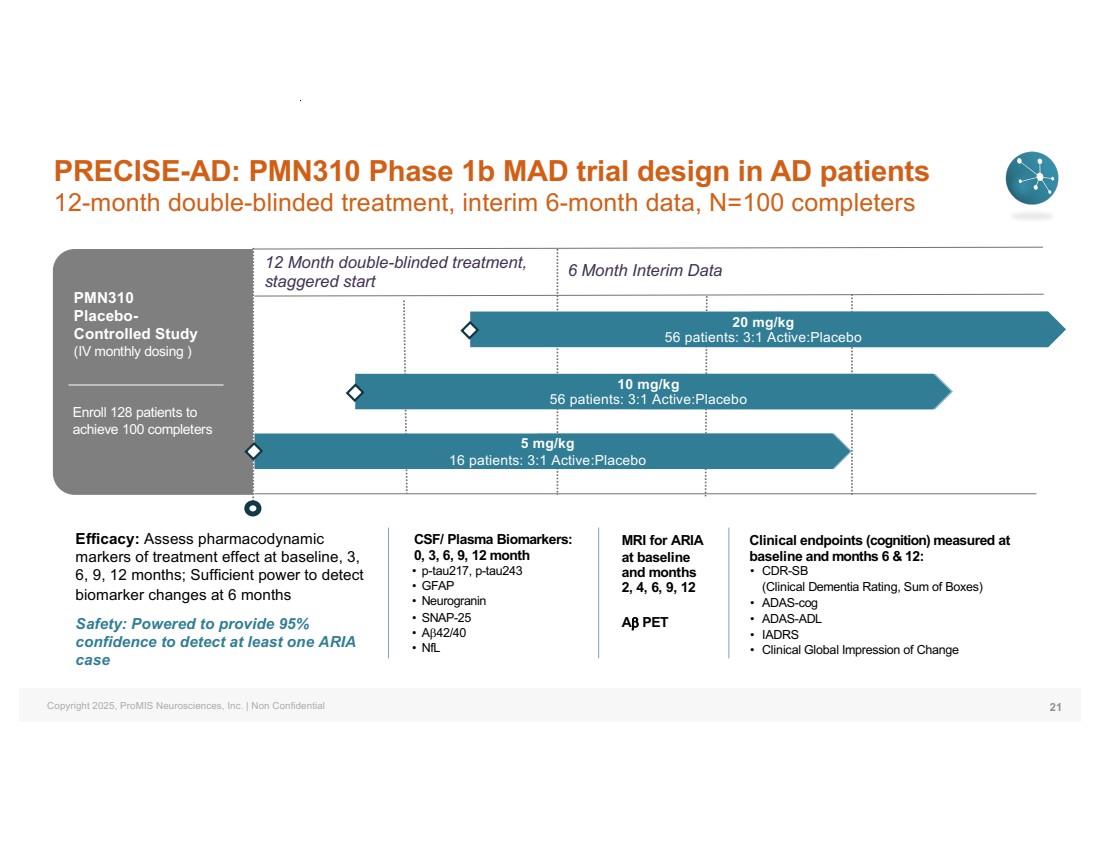
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Clinical endpoints (cognition) measured at baseline and months 6 & 12: • CDR-SB (Clinical Dementia Rating, Sum of Boxes) • ADAS-cog • ADAS-ADL • IADRS • Clinical Global Impression of Change CSF/ Plasma Biomarkers: 0, 3, 6, 9, 12 month • p-tau217, p-tau243 • GFAP • Neurogranin • SNAP-25 • Ab42/40 • NfL Efficacy: Assess pharmacodynamic markers of treatment effect at baseline, 3, 6, 9, 12 months; Sufficient power to detect biomarker changes at 6 months Safety: Powered to provide 95% confidence to detect at least one ARIA case MRI for ARIA at baseline and months 2, 4, 6, 9, 12 Ab PET PRECISE-AD: PMN310 Phase 1b MAD trial design in AD patients 12-month double-blinded treatment, interim 6-month data, N=100 completers 12 Month double-blinded treatment, staggered start 5 mg/kg 16 patients: 3:1 Active:Placebo 10 mg/kg 56 patients: 3:1 Active:Placebo 20 mg/kg 56 patients: 3:1 Active:Placebo Enroll 128 patients to achieve 100 completers 6 Month Interim Data PMN310 Placebo-Controlled Study (IV monthly dosing ) 21 |
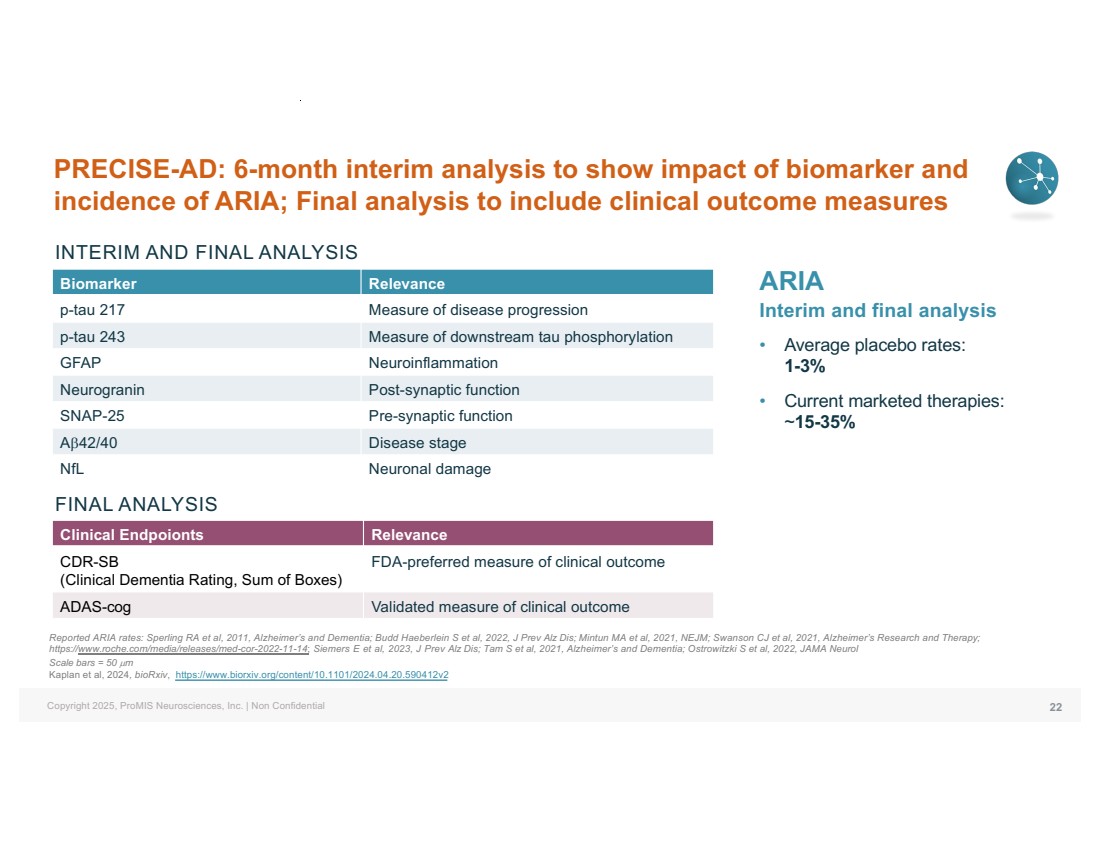
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PRECISE-AD: 6-month interim analysis to show impact of biomarker and incidence of ARIA; Final analysis to include clinical outcome measures Biomarker Relevance p-tau 217 Measure of disease progression p-tau 243 Measure of downstream tau phosphorylation GFAP Neuroinflammation Neurogranin Post-synaptic function SNAP-25 Pre-synaptic function Ab42/40 Disease stage NfL Neuronal damage 22 Clinical Endpoionts Relevance CDR-SB (Clinical Dementia Rating, Sum of Boxes) FDA-preferred measure of clinical outcome ADAS-cog Validated measure of clinical outcome INTERIM AND FINAL ANALYSIS FINAL ANALYSIS ARIA Interim and final analysis • Average placebo rates: 1-3% • Current marketed therapies: ~15-35% Reported ARIA rates: Sperling RA et al, 2011, Alzheimer’s and Dementia; Budd Haeberlein S et al, 2022, J Prev Alz Dis; Mintun MA et al, 2021, NEJM; Swanson CJ et al, 2021, Alzheimer’s Research and Therapy; https://www.roche.com/media/releases/med-cor-2022-11-14; Siemers E et al, 2023, J Prev Alz Dis; Tam S et al, 2021, Alzheimer’s and Dementia; Ostrowitzki S et al, 2022, JAMA Neurol Scale bars = 50 µm Kaplan et al, 2024, bioRxiv, https://www.biorxiv.org/content/10.1101/2024.04.20.590412v2 |
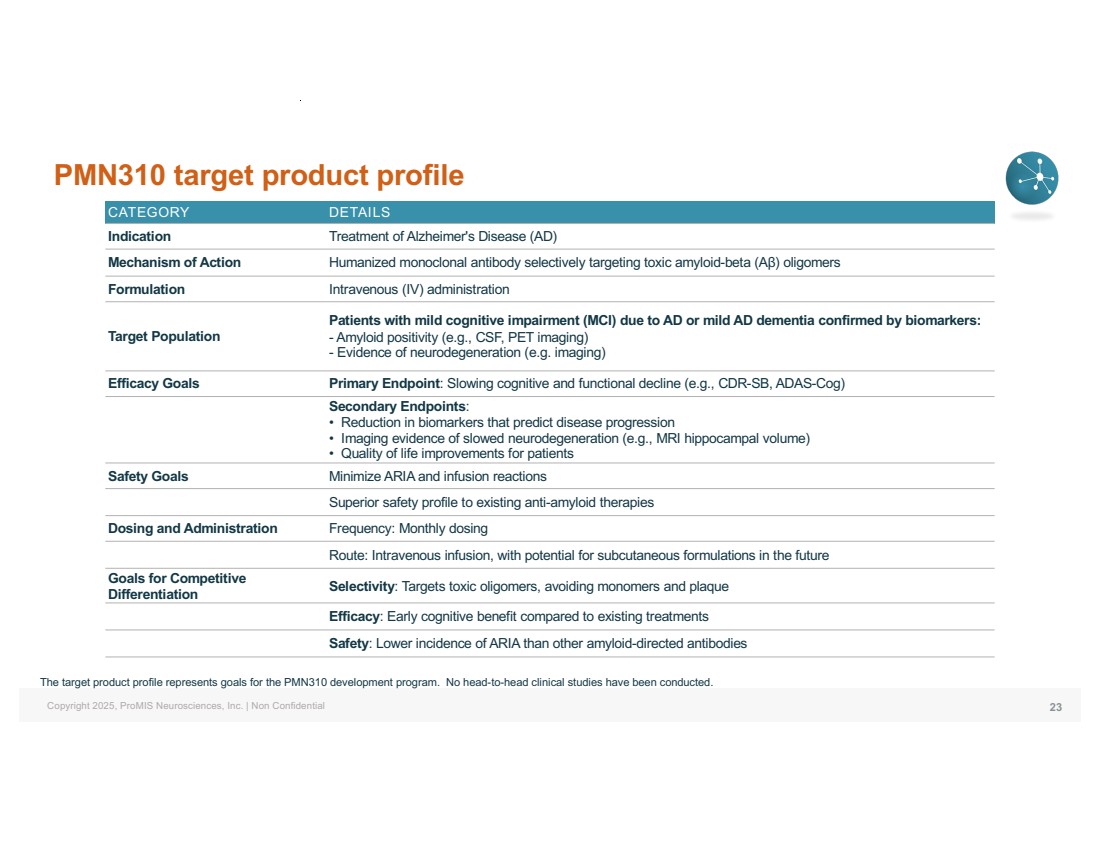
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PMN310 target product profile 23 CATEGORY DETAILS Indication Treatment of Alzheimer's Disease (AD) Mechanism of Action Humanized monoclonal antibody selectively targeting toxic amyloid-beta (Aβ) oligomers Formulation Intravenous (IV) administration Target Population Patients with mild cognitive impairment (MCI) due to AD or mild AD dementia confirmed by biomarkers: - Amyloid positivity (e.g., CSF, PET imaging) - Evidence of neurodegeneration (e.g. imaging) Efficacy Goals Primary Endpoint: Slowing cognitive and functional decline (e.g., CDR-SB, ADAS-Cog) Secondary Endpoints: • Reduction in biomarkers that predict disease progression • Imaging evidence of slowed neurodegeneration (e.g., MRI hippocampal volume) • Quality of life improvements for patients Safety Goals Minimize ARIA and infusion reactions Superior safety profile to existing anti-amyloid therapies Dosing and Administration Frequency: Monthly dosing Route: Intravenous infusion, with potential for subcutaneous formulations in the future Goals for Competitive Differentiation Selectivity: Targets toxic oligomers, avoiding monomers and plaque Efficacy: Early cognitive benefit compared to existing treatments Safety: Lower incidence of ARIA than other amyloid-directed antibodies The target product profile represents goals for the PMN310 development program. No head-to-head clinical studies have been conducted. |
| Lead Pipeline Candidates Antibody and Vaccine Candidates Targeting a Range of Neurodegenerative Diseases |
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PMN267: Lead antibody candidate targeting pathogenic TDP-43 25 Why TDP-43? • TAR DNA-binding protein 43 (TDP-43) essential to neuronal cell survival1; plays important roles in RNA regulation • Pathogenic TDP-43 aggregates frequently observed in multiple neurodegenerative diseases: both loss-of-function1 and gain of function2 PMN267 – Initial proof of concept to target Amyotrophic Lateral Sclerosis (ALS) • Target candidate profile specific for binding epitope of pathogenic TDP-43 with high affinity in sub-nanomolar range. No reactivity with normal TDP-43. • Inhibit cell-to-cell propagation of toxic misfolded TDP-43 • Promote degradation of cytoplasmic aggregates of misfolded TDP-43 without affecting cell viability 1. de Boer, EMJ et al, 2020, J Neurol Neurosurg Psychiatry; 2. Neumann et al, 2006, Science; 3. Pokrishevsky et al, 2016, Scientific Reports; 4. Chou et al, 2018, Nat Neurosci; 5. Endo et al, 2018, Biological Psych TDP-43 Healthy (motor) neuron Degenerating ALS/FTD (motor) neuron |
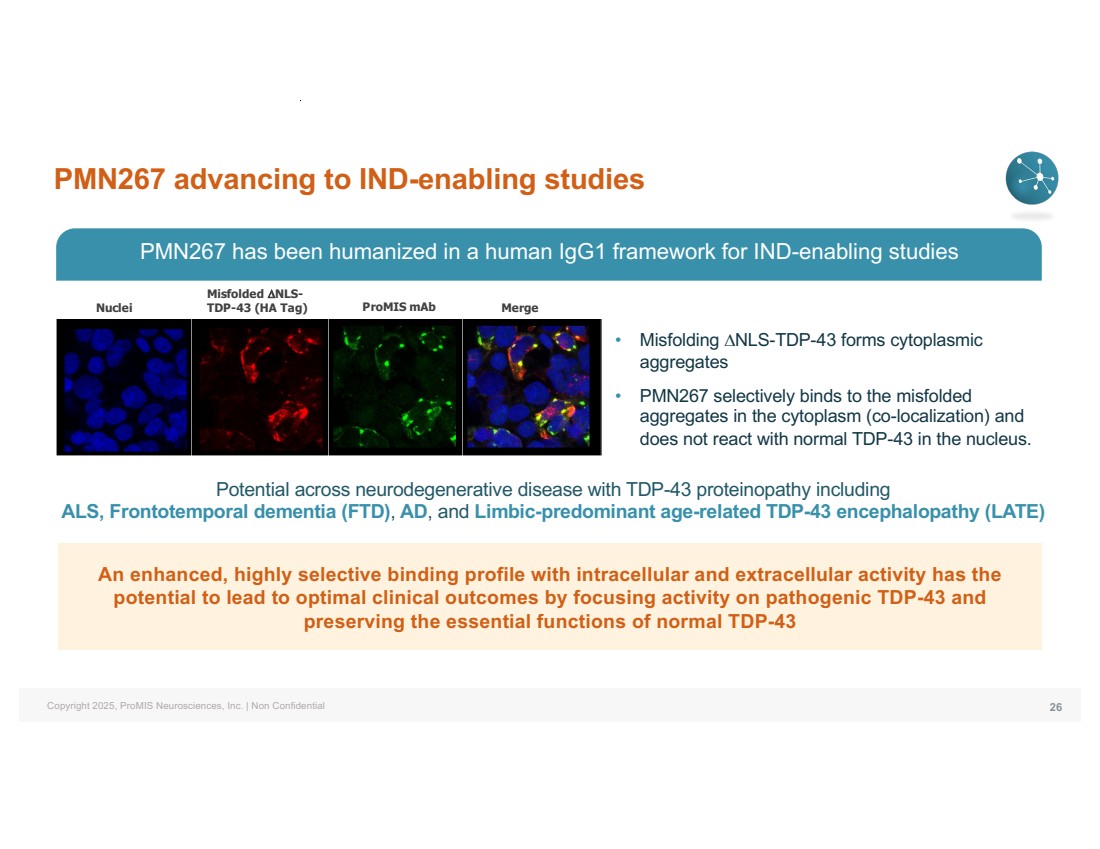
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PMN267 advancing to IND-enabling studies 26 Nuclei Misfolded DNLS-TDP-43 (HA Tag) ProMIS mAb Merge Potential across neurodegenerative disease with TDP-43 proteinopathy including ALS, Frontotemporal dementia (FTD), AD, and Limbic-predominant age-related TDP-43 encephalopathy (LATE) • Misfolding DNLS-TDP-43 forms cytoplasmic aggregates • PMN267 selectively binds to the misfolded aggregates in the cytoplasm (co-localization) and does not react with normal TDP-43 in the nucleus. An enhanced, highly selective binding profile with intracellular and extracellular activity has the potential to lead to optimal clinical outcomes by focusing activity on pathogenic TDP-43 and preserving the essential functions of normal TDP-43 PMN267 has been humanized in a human IgG1 framework for IND-enabling studies |
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PMN442: Lead antibody candidate targeting toxic α-Synuclein 27 Why Alpha-Synuclein (α-syn)? • α-syn plays a role in synaptic activity, including regulating release of dopamine and maintaining synaptic vesicles • In synucleinopathies, α-syn misfolds and clumps into toxic aggregates implicated in: Multiple system atrophy (MSA), Parkinson’s Disease (PD), and Dementia with Lewy bodies (DLB) PMN442 – Initial Proof of Concept to Target Multiple System Atrophy (MSA) • Target candidate profile specific for toxic oligomers and small soluble fibrils, avoiding monomers and tetramers to reduce potential adverse events • Protect dopaminergic neurons against killing by α-syn toxic oligomers • Inhibit the processes involved in the cell-to-cell propagation of pathogenic α-syn aggregates Pathogenic α-syn Neuronal uptake Neurodegeneration Diseased neuron Microglial activation |
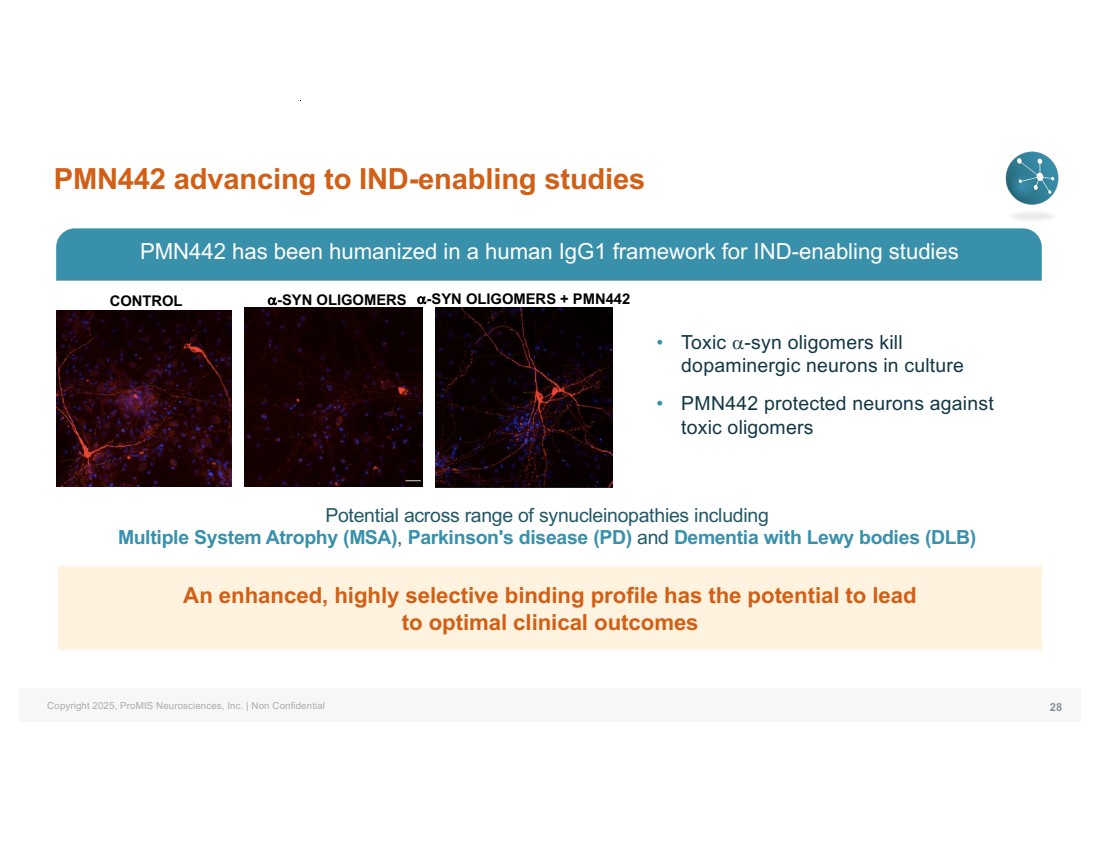
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PMN442 advancing to IND-enabling studies 28 CONTROL a-SYN OLIGOMERS a-SYN OLIGOMERS + PMN442 PMN442 has been humanized in a human IgG1 framework for IND-enabling studies Potential across range of synucleinopathies including Multiple System Atrophy (MSA), Parkinson's disease (PD) and Dementia with Lewy bodies (DLB) • Toxic a-syn oligomers kill dopaminergic neurons in culture • PMN442 protected neurons against toxic oligomers An enhanced, highly selective binding profile has the potential to lead to optimal clinical outcomes |
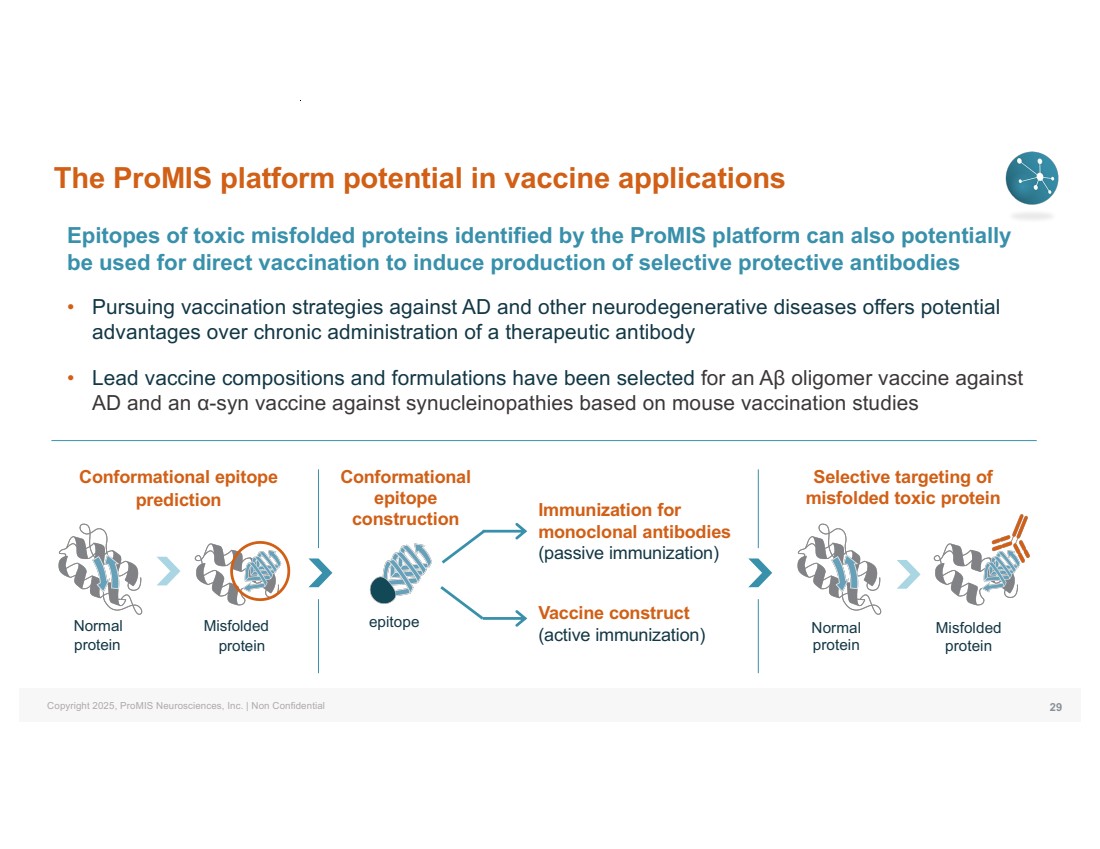
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential The ProMIS platform potential in vaccine applications Epitopes of toxic misfolded proteins identified by the ProMIS platform can also potentially be used for direct vaccination to induce production of selective protective antibodies • Pursuing vaccination strategies against AD and other neurodegenerative diseases offers potential advantages over chronic administration of a therapeutic antibody • Lead vaccine compositions and formulations have been selected for an Aβ oligomer vaccine against AD and an α-syn vaccine against synucleinopathies based on mouse vaccination studies 29 Conformational epitope prediction Conformational epitope construction Normal protein Misfolded protein Selective targeting of misfolded toxic protein Immunization for monoclonal antibodies (passive immunization) epitope Normal protein Misfolded protein Vaccine construct (active immunization) |
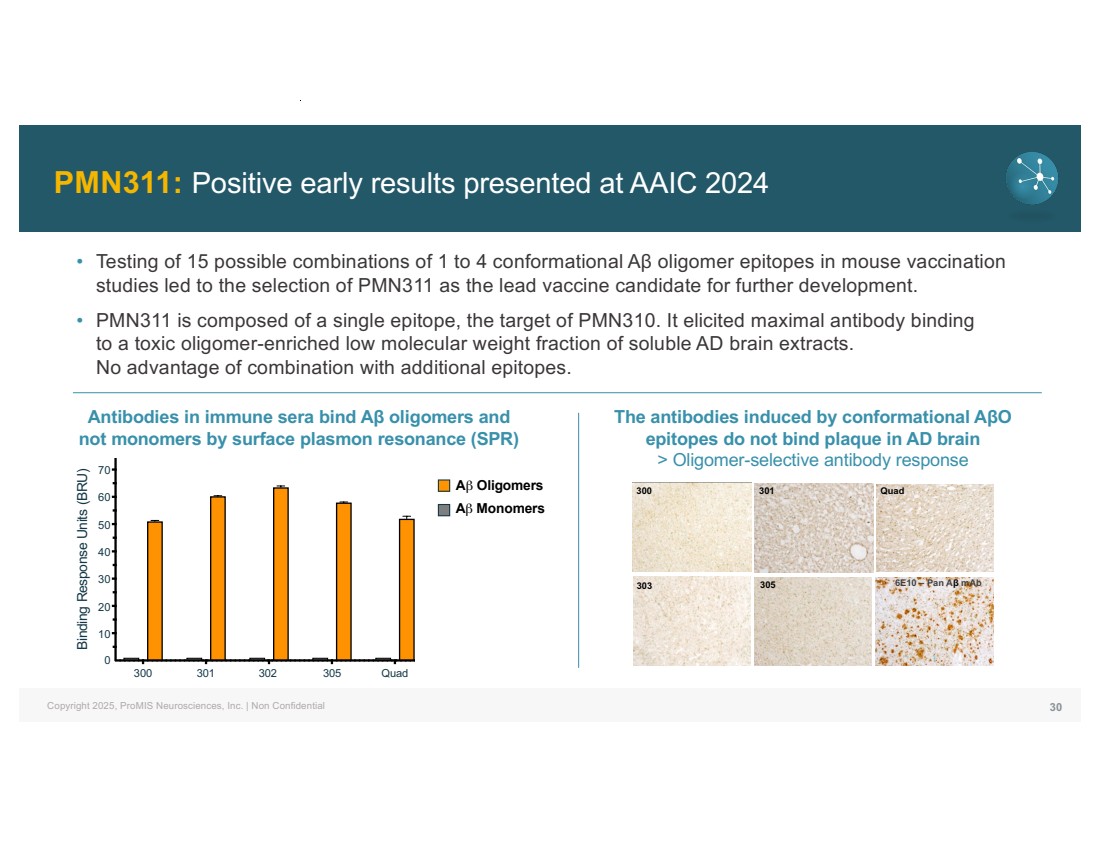
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential PMN311: Positive early results presented at AAIC 2024 30 • Testing of 15 possible combinations of 1 to 4 conformational Aβ oligomer epitopes in mouse vaccination studies led to the selection of PMN311 as the lead vaccine candidate for further development. • PMN311 is composed of a single epitope, the target of PMN310. It elicited maximal antibody binding to a toxic oligomer-enriched low molecular weight fraction of soluble AD brain extracts. No advantage of combination with additional epitopes. 300 301 303 305 Quad 6E10 – Pan Aβ mAb Antibodies in immune sera bind Aβ oligomers and not monomers by surface plasmon resonance (SPR) The antibodies induced by conformational AβO epitopes do not bind plaque in AD brain > Oligomer-selective antibody response 300 301 303 305 Quad 0 10 20 30 40 50 60 70 Binding Response Units (BRU) Aβ Oligomers Aβ Monomers 300 301 303 305 Quad 0 10 20 30 40 50 60 70 Binding Response Units (BRU) Aβ Oligomers Aβ Monomers 300 301 302 305 Quad 0 10 20 30 40 50 60 70 Binding Response Units (BRU) |
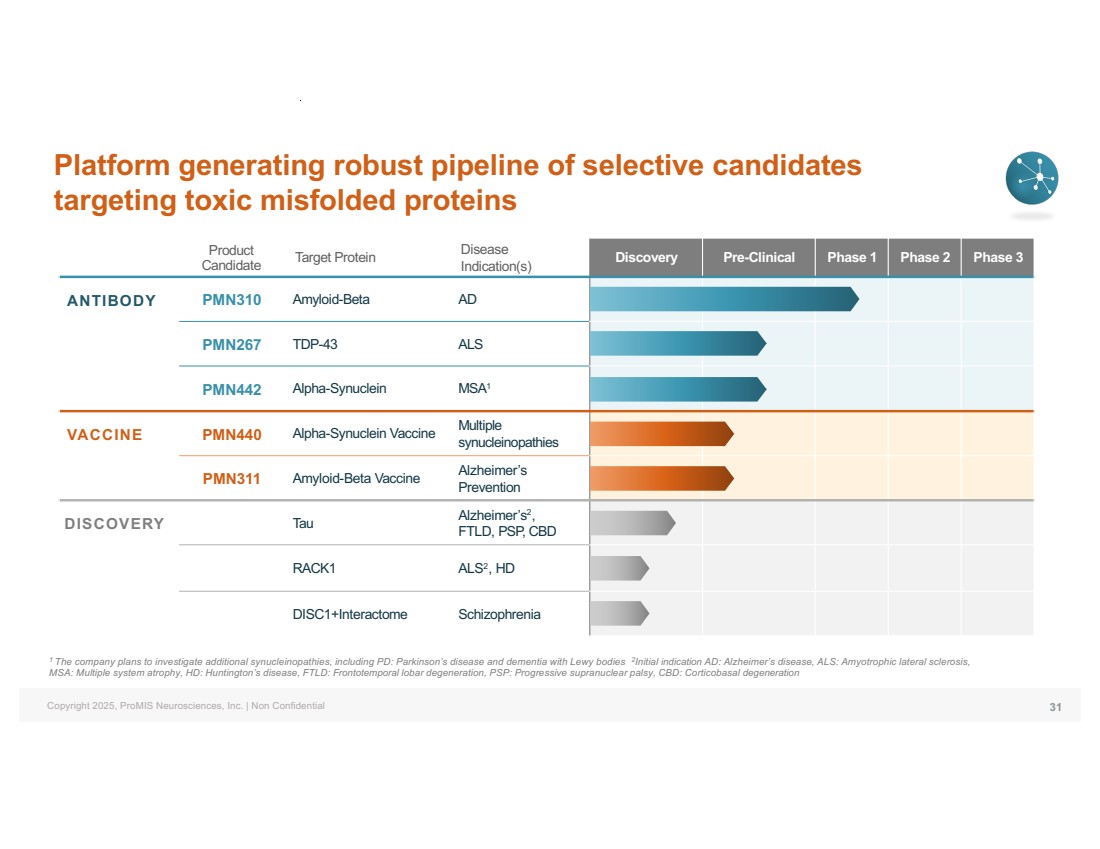
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Product Candidate Target Protein Disease Indication(s) Discovery Pre-Clinical Phase 1 Phase 2 Phase 3 ANTIBODY PMN310 Amyloid-Beta AD PMN267 TDP-43 ALS PMN442 Alpha-Synuclein MSA1 VACCINE PMN440 Alpha-Synuclein Vaccine Multiple synucleinopathies PMN311 Amyloid-Beta Vaccine Alzheimer’s Prevention DISCOVERY Tau Alzheimer’s2, FTLD, PSP, CBD RACK1 ALS2, HD DISC1+Interactome Schizophrenia 1 The company plans to investigate additional synucleinopathies, including PD: Parkinson’s disease and dementia with Lewy bodies 2Initial indication AD: Alzheimer’s disease, ALS: Amyotrophic lateral sclerosis, MSA: Multiple system atrophy, HD: Huntington’s disease, FTLD: Frontotemporal lobar degeneration, PSP: Progressive supranuclear palsy, CBD: Corticobasal degeneration Platform generating robust pipeline of selective candidates targeting toxic misfolded proteins 31 |
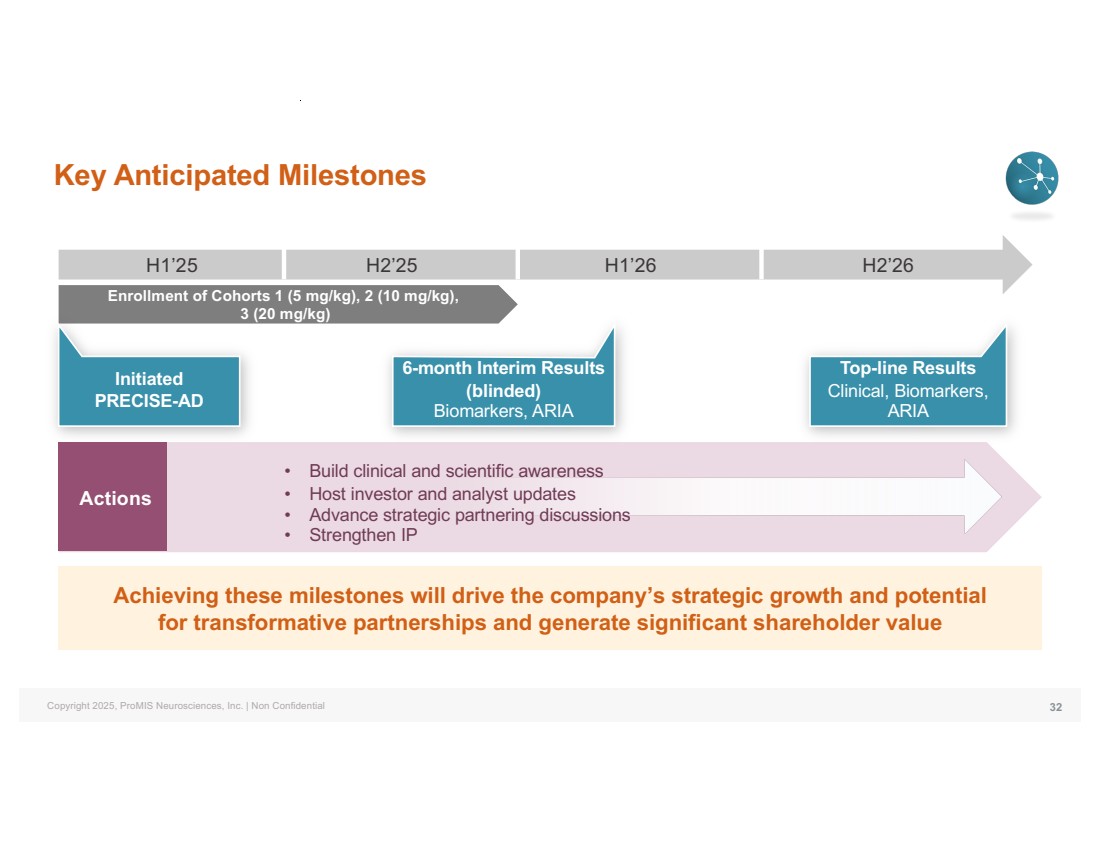
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Enrollment of Cohorts 1 (5 mg/kg), 2 (10 mg/kg), 3 (20 mg/kg) Initiated PRECISE-AD Key Anticipated Milestones 32 H1’25 H2’25 H1’26 H2’26 Achieving these milestones will drive the company’s strategic growth and potential for transformative partnerships and generate significant shareholder value Actions • Build clinical and scientific awareness • Host investor and analyst updates • Advance strategic partnering discussions • Strengthen IP 6-month Interim Results (blinded) Biomarkers, ARIA Top-line Results Clinical, Biomarkers, ARIA |
| Copyright 2025, ProMIS Neurosciences, Inc. | Non Confidential Committed to patients with novel approach to battling neurodegenerative diseases 33 Clinical data and milestones could unlock significant potential and demonstrate proof of concept for PMN310 in AD Advancing preclinical pipeline could further validate the ProMIS platform and the potential across therapeutics and vaccines Strong track record of execution and seasoned leadership team with significant CNS product development experience Committed financing supports programs through key inflection points ProMIS has leveraged AI/ML to create a novel technology platform that has generated a robust pipeline of candidates against Alzheimer’s, ALS, MSA and other challenging diseases Clinical candidate PMN310 highly differentiated; data from ongoing Phase 1b clinical trial aims to evaluate safety and tolerability to assess PMN310’s potential to halt Alzheimer’s disease progression |
| NASDAQ: PMN For further information, contact: info@promisneurosciences.com |